Atom is a basic unit of matter, which consists of atomic nuclei and negatively charged electron cloud surrounding it. Atomic nucleus consists of positively charged protons and electrically neutral neutrons (except hydrogen-1 nucleus, which has no neutrons). The electrons in an atom bound to the nucleus by the electromagnetic force. As well as a collection of atoms can bind to each other, and form a molecule. Atoms containing the number of protons and electrons are equal are neutral, while containing the number of protons and electrons of different positive or negative and is referred to as ion. Atoms are grouped based on the number of protons and neutrons contained in the nucleus.
The number of protons in an atom determines the chemical element the atom, and the number of neutrons determines the isotope of the element. The term comes from the Greek atom (ἄτομος / Atomos, α-τεμνω), which means it can not be cut or something that can not be divided again. Atomic concept as a component that can not be divided again was first proposed by the Indian and Greek philosophers. In the 17th century and into the 18th, the chemists laid the foundations of this idea by showing that certain substances can not be divided further using chemical methods. During the late 19th century and early 20th century, physicists have managed to find the structure and subatomic components inside the atom, to prove that the 'atom' is not can not be divided again. The principles of quantum mechanics used by physicists then successfully model the atom.
In day-to-day observations, the relative atomic considered a very small objects that have mass proportionally small anyway. Atoms can only be monitored using special equipment such as atomic force microscopy. More than 99.9% of the mass of the atom is concentrated in the nucleus, with protons and neutrons are nearly the same mass. Each element has at least one isotope with unstable nuclei that can undergo radioactive decay. This can result in transmutation, which changes the number of protons and neutrons in the nucleus.
Electrons are bound to the atom contains a number of energy levels, or orbitals, which is stable and can undergo transitions between those levels by absorbing or emitting photons that match the energy differences between the levels. Electrons in an atom determines the chemical properties of an element, and affects the magnetic properties of the atom.
History
The concept that matter is composed of separate units that can not be subdivided into smaller units has existed for over a millennium. However, these ideas were founded in abstract, philosophical, rather than based on empirical observations and experiments. Philosophically, the description of the properties of atoms varies depending on the culture and philosophy of the stream, and often had spiritual elements in it. Nevertheless, the basic ideas of the atom can be accepted by scientists thousands of years later, because he can elegantly explained new discoveries in the field of chemistry.
The earliest reference of the concept of the atom can be traced back to ancient India in the year 800 BC, which is described in the script as such and Jainism philosophy paramanu. Flow Nyaya and Vaisesika school developed a theory that explains how atoms combine into objects that are more complex. A century later references to atoms emerged in the Western world by Leukippos, which later by his pupil Democritus that view systematised.
Approximately in the year 450 BCE, Democritus coined the term atomos (Greek: ἄτομος), which means "can not cut" or "can not be divided again." Theory of Democritus about atoms is not an attempt to describe a physical phenomenon in detail, but rather a philosophy that tries to provide answers to the changes that occur in nature. Philosophy is also common in India, however, modern science decided to use the term "atom" is coined by Democritus.
Further progress in the understanding of the atom begins with the development of chemistry. In 1661, Robert Boyle published The Sceptical Chymist who argued that the material world is made up of various combinations of "corpuscules", ie different atoms. This is in contrast with the classical view that argues that matter is composed of elements of air, earth, fire, and water. In 1789, the term element (element) is defined by the French nobleman and researcher, Antoine Lavoisier, as the base material which can not be divided further by using chemical methods.
In 1803, John Dalton used the concept of atoms to explain why elements always react in the comparison round and fixed, and why certain gases are more soluble in water than other gases. He proposed that each element consists of a unique single atoms, and the atoms can then combine to form chemical compounds.
Particle theory is then further confirmed in 1827, when Robert Brown botaniwan use a microscope to observe the dust that floats on the water and found that the dust is moving randomly. This phenomenon became known as "Brownian motion". In 1877, J. Desaulx propose that this phenomenon is caused by the thermal motion of water molecules, and in 1905 Albert Einstein made a mathematical analysis on this motion. French physicist Jean Perrin then use Einstein's work to determine the mass and dimensions of atoms in the experiment, which later became the definitive verification of Dalton's atomic theory.
Based on the results of his research on cathode rays, in 1897 J. J. Thomson discovered the electron and subatomiknya properties. This demolishes the concept of the atom as a unit which can not be divided again. Thomson believed that the electrons are distributed evenly throughout the atom, and the charge-balanced by the presence ocean cargo positive charge (the plum pudding model).
But in 1909, researchers under the direction of Ernest Rutherford fired helium ions into thin sheets of gold, and found that the ion fraction reflected by the reflection angle sharper than what is predicted by the theory of Thomson. Rutherford then proposed that the positive charge of an atom and most of its mass concentrated in the nucleus, with electrons orbiting the nucleus like planets around the sun. Helium ion positive charge that passes through the dense core must be reflected by the reflection of a sharper angle. In 1913, while experimenting with the result of radioactive decay, Frederick Soddy discovered that there is more than one kind of atom at each position on the periodic table. The term isotope then created by Margaret Todd as a proper name for different atoms but it is the same element. J.J. Thomson subsequently found the technique for separating atom types through his work on ionized gases.
Meanwhile, in 1913 reviewed the physicist Niels Bohr and Rutherford atomic model proposed that electrons lies in quantized orbits, and could jump from one orbit to another orbit, though not freely rotating spiral into and out in a state of transition. An electron must absorb or emit specific amounts of energy to be able to make the transition between fixed orbits this. When light from a heated material radiates through a prism, it produces a multicolored spectrum. The appearance of certain spectral lines is successfully explained by these orbital transitions theory.
Chemical bonds between atoms and then in 1916 by Gilbert Newton Lewis described as the interaction between the electron-electron atom. On the regularity of the chemical properties of the chemical periodic table, the American chemist Irving Langmuir in 1919 argues that this can be explained if the electrons in an atom or interconnected together in certain forms. A group is expected to occupy a set of electron sheath electrons around the atomic nucleus.
Stern-Gerlach experiment in 1922 provided further evidence of the quantum properties of atoms. When a beam of silver atoms were fired through a magnetic field, the file separately in accordance with the direction of the atomic angular momentum (spin). Therefore, the spin direction is random, the file is expected to spread into a line. But in fact the file is divided into two parts, depending on whether the atomic spin oriented up or down.
In 1926, using Louis de Broglie thought that the particles behave like waves, Erwin Schrödinger developed a mathematical model of the atom describe electrons as waves rather than as a three-dimensional particle dots.
Consequence of using waveforms to describe electrons this is that it is not possible to mathematically calculate the position and momentum of a particle simultaneously.
This became known as the uncertainty principle, formulated by Werner Heisenberg in 1926. According to this concept, for each measurement position, one could only obtain a range of probable values for momentum, and vice versa. Although this model is difficult to visualize, it can well explain the observed properties of atoms that previously could not be explained by any theory. Therefore, the model that describes the electrons surrounding the atomic nucleus like planets around the sun were dropped and replaced by a model of the atomic orbitals around the nucleus where electrons are most likely to be.
Developments in mass spectrometry permitted a precise measurement of the mass of the atom. This equipment uses a magnetic spectrometer to deflect the trajectory of the ion beam, and the amount of deflection is determined by the ratio of the atomic mass of the payload. Chemist Francis William Aston to use this equipment to show that isotopes had different masses. Mass difference between isotopes is an integer, and it is referred to as the rules of integers. The explanation for this difference in isotopic masses awaited the discovery of the neutron, an electrically neutral particle with a mass similar to the proton, ie by James Chadwick in 1932. Isotopes were then explained as elements with the same number of protons, but different numbers of neutrons in the atomic nucleus.
In the 1950s, the development of particle accelerators and particle detectors allowed scientists study the impacts of atoms moving at high energies. Neutrons and protons then known as hadrons, namely composite tiny particles called quarks. Standard models of nuclear physics were then developed to explain the properties of atomic nuclei in terms of the interaction of subatomic particles.
Around 1985, Steven Chu et al. at Bell Labs developed a technique to reduce the temperature of atoms using lasers. In the same year, a group led by William D. Phillips managed to trap sodium atoms in a magnetic trap. Claude Cohen-Tannoudji and then combine these two techniques to cool a small number of atoms to a few microkelvins. This allows scientists to study the atomic precision is very high, which in turn brings the scientists found the Bose-Einstein condensation.
Historically, a single atom is very small for use in scientific applications. But recently, a variety of devices that use a single metal atom connected by organic ligands (single electron transistor) has been made. Various studies have been conducted to trap and slow the pace of atoms using laser cooling to obtain a better understanding of the properties of atoms.
Atomic Components Sub-Atomic Particles
Although initially the word atom means a particle that can not be cut again into smaller particles, in modern scientific terminology, atom is composed of various subatomic particles. Subatomic particles are electrons, protons, and neutrons. However, hydrogen-1 has no neutrons. Similarly, the positive hydrogen ion H +.
Of all the subatomic particles, electrons are the lightest, with the mass of the electron by 9.11 × 10-31 kg and has a negative charge. Electron size is very small so universally no measurement techniques that can be used to measure its size. Protons have a positive charge and a mass 1,836 times heavier than an electron (1.6726 × 10-27 kg). Neutrons have no electrical charge and have a free mass of 1,839 times the mass of the electron or (1.6929 × 10-27 kg).
In the standard model of physics, both protons and neutrons are composed of elementary particles called quarks. Quark belongs to the fermion group of particles and is one of the two basic building blocks of matter (the other is the lepton). There are six types of quarks and each quark has a fractional charge electricity at +2 / 3 or -1 / 3. Proton consists of two up quarks and one down quark, when the neutron consists of one up quark and two down quarks.
This distinction affects the difference in mass and charge between the two particles. Quarks bound together by the strong nuclear force mediated by gluons. Gluon is a member of the gauge bosons which are intermediaries physical forces.
Core Atom
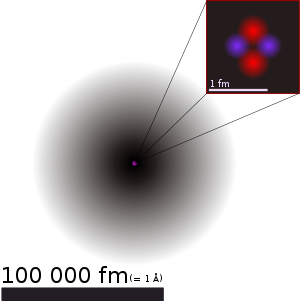
Atomic nucleus consists of protons and neutrons are bound together in the center of the atom. Collectively, protons and neutrons are called nucleons (particles making up the nucleus). Nucleus diameter ranging between 10-15 and 10-14m. Estimated core radii equal to \ begin {smallmatrix} 1.07 \ sqrt [3] {A} \ end {smallmatrix} fm, where A is the number of nucleons. This is much smaller than the atomic radius. Nucleons are bound together by the force of attraction of potential called the residual strong force. At distances smaller than 2.5 fm, the force is stronger than the electrostatic force that causes protons repel each other.
Atoms of the same element have the same number of protons, called the atomic number. An element can have varying numbers of neutrons. Variations in the isotope. Number of protons and neutrons of an atom determines the atomic nuclides, while the number of neutrons relative to the number of protons determines the stability of the nucleus, with certain isotopes will run radioactive decay.
Neutrons and protons are two different types of fermions. Pauli exclusion principle prohibits the existence of identical fermions (such as multiple protons) occupying the same quantum physical state at the same time. Therefore, every proton in the nucleus of an atom should occupy different quantum states with energy levels, respectively. The Pauli principle is also applicable to neutrons. This prohibition does not apply to the proton and neutron occupying the same quantum state.
For atoms with low atomic number, atomic nuclei that have more than the number of protons neutrons could potentially fall into a lower energy state through a radioactive decay that causes the number of protons and neutrons balanced. Therefore, the atom the number of protons and neutrons are more stable and balanced tend not decay. However, with increasing atomic number, repulsion between protons makes the nucleus requires a higher proportion of neutrons to maintain the stability again. At the core of the most severe, the ratio of neutrons per proton required to maintain the stability will increase to 1.5.
The number of protons and neutrons in the atomic nucleus can be modified, although this can require very high energies because of the strong force. Nuclear fusion occurs when many particles are atoms combine to form a heavier nucleus. For example, at the core of the Sun protons require approximately 3-10 keV energy to overcome the repulsion between each other and merge into a single core. Nuclear fission is the opposite of the fusion process. On nuclear fission, the nucleus split into two smaller nuclei. This usually occurs through radioactive decay. Nucleus can also be modified through bombardment by high energy subatomic particles. If this is to change the number of protons in the nucleus, the atom will change the element.
If the core mass after the fusion reaction is smaller than the sum of its constituent particles mass start, then the difference is caused by the release of radiant energy (such as gamma rays), as found in the formula of Einstein's mass-energy equivalence, E = mc2, where m is the mass of the lost and c is the speed of light. This deficit is part of the new core binding energy.
Fusion of two nuclei that produce larger nuclei with lower atomic numbers than iron and nickel (total number of nucleons with 60) normally is exothermic, which means that this process releases energy. Is the energy-releasing process that makes nuclear fusion in stars can be maintained. For heavier nuclei, the binding energy per nucleon in the nucleus begins to decrease. This means that the fusion process will be endothermic.
Electron cloud Electrons in an atom is pulled by a proton in the nucleus through the electromagnetic force. This force binds the electrons inside an electrostatic potential well around the core. This means that the external energy needed for the electrons to escape from the atom. The closer an electron in the nucleus, the greater the attraction force, so that the electrons are located near the center of the potential well require more energy to escape.
Electrons, like other particles, have properties such as particle or as a wave (wave-particle duality). Electron cloud is a region inside the potential well where each electron produces a type of stationary waves (ie waves that do not move relative to the nucleus) three-dimensional. This behavior is determined by the atomic orbitals, which is a mathematical function that calculates the probability that an electron will appear at a particular location when its position is measured. There will be only one set of specific orbitals exist around the nucleus, as other possible wave patterns will rapidly decay into a more stable form.
Each atomic orbital corresponds to a particular electron energy levels. Electrons can change the situation to a higher energy level by absorbing a photon. Besides being able to go up to the higher energy levels, an electron can also go down to a lower energy state by emitting the excess energy as photons.
Energy needed to remove or add an electron (electron binding energy) is smaller than the binding energy of nucleons. For example, only 13.6 eV is required to remove an electron from the hydrogen atom. Compare the energy of 2.3 MeV are needed to break the deuterium nucleus. Atoms are electrically neutral because the number of protons and electrons are the same. Atoms that lack or excess of electrons called ions. Electrons are located outside of the core can be shared or transferred to other nearby atoms. In this way, atoms can bond together to form molecules.
Properties
Nuclear properties
By definition, the two atoms with an identical number of protons in their nuclei belong to the same chemical element. Atoms with the same number of protons but different numbers of neutrons are the two different isotopes of the same element. For example, all of the hydrogen has one proton, but there is an isotope of hydrogen that has no neutrons (hydrogen-1), an isotope that has one neutron (deuterium), two neutrons (tritium), etc.. Hydrogen-1 is the form of the most common isotope of hydrogen. Sometimes he is referred to as protium. All isotopes of elements atomic number greater than 82 are radioactive.
Of approximately 339 naturally occurring nuclides on Earth, 269 of which have never been observed to decay. In the chemical elements, 80 of which are known element has one or more stable isotopes. Elements 43, 63, and all elements higher than 83 have no stable isotopes. Twenty-seven elements have only one stable isotope, when the number of stable isotopes most widely observed in the element tin with 10 stable isotopes.
Mass
Because the majority of the mass of an atom comes from the protons and neutrons, the total number of particles in an atom is called the mass number. Atomic mass at rest is often expressed using atomic mass unit (u) is also called the dalton (Da). This unit is defined as one-twelfth the mass of a neutral atom of carbon-12, which is approximately 1.66 × 10-27 kg. Hydrogen-1 which is the lightest hydrogen isotope has atomic weight of 1.007825 u. Atom has a mass approximately equal to the mass number atomic mass unit. The heaviest stable atom is lead-208, with a mass of 207.9766521 u.
The chemists typically use a unit to express the number of moles of atoms. One mole is defined as the number of atoms contained in exactly 12 grams of carbon-12. This number is approximately 6.022 × 1023, which is also known as the Avogadro constant. Thus an element with the atomic mass of 1 u will have a mass of one mole of atoms is 0.001 kg. For example, Carbon has an atomic mass 12 u, so one mole of carbon atoms has a mass of 0,012 kg.
Size
Atom does not have a clear outer boundary, so that the dimensions of atoms is usually described as the distance between the two nuclei when the two atoms joined together in a chemical bond. The radius varies depending on the type of atoms, types of bonds involved, the number of atoms in the vicinity, and the atomic spin. In the periodic table of elements, atomic radius will tend to increase with increasing period (top to bottom). In contrast to the atomic radius tends to increase with decreasing group numbers (right to left). Therefore, the smallest atom is helium with a radius of 32 pm, when the largest is cesium at 225 pm radius. These dimensions are thousands of times smaller than the wavelength of light (400-700 nm), so that the atoms can not be seen using ordinary optical microscope. However, atoms can be monitored using atomic force microscopy.
Atomic size is very small, so small width of one human hair is about 1 million carbon atoms. Similarly one drop of water contains about 2 × 1021 atoms of oxygen. One carat diamond with a mass of 2 × 10-4 kg contains about 1022 carbon atoms. If an apple is magnified to the size of the magnitude of the Earth, then the atoms in the apple would be approximately the size of the early apples.
Sources: http://id.wikipedia.org/wiki/Atom

No comments:
Post a Comment